C2+ C2- - Chemistry Community
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to other diatomic molecules, but involving more than the 1s atomic orbitals. For the second period elements, the 2s and 2p orbitals are important for MO considerations. A linear combination of properly oriented atomic orbitals for the formation of sigma s and pi p bonds.When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...C2 molecular orbital diagram. As you can see in the diagram the two 2ppi orbitals lets say 2ppix and 2ppiy are the highest energy occupied molecular orbitals. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization. Molecular orbital diagram for oxygen gas o2.The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons.
Molecular Orbitals of Li₂ to F₂ - Chemistry LibreTexts
Molecular orbital diagram for carbon dimer c2. C2 2 molecular orbital diagram. H he li be b c n o f ne b c n o f ne na mg al si p s cl ar al si p s cl ar 1s 2s 2p 3s 136 ev 3p. The molecular orbital diagram for c22 this problem has been solved. Bonding order is 2 and it is diamagnetic. The molecular orbital diagram for c22 question. This videomolecular orbital diagram for C2. number of electrons in the sigma2p molecular orbital is. 0. molecular orbital diagram for N2. number of electrons in the sigma2p molecular orbital is. 2. molecular orbital diagram for O2. number of elections in the pi*2p molecular orbital is. 1.Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+.Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than

Molecular Orbital (MO) Diagram for C2(2-) - YouTube
This diagram should be to scale. If necessary, you can omit the two lowest molecular orbitals from the diagram. Write the molecular orbital configuration for your molecule. Calculate the bond order for your molecule. Jen B2 Jeff OH- Mike F C2 Laura Li2 Jill Be2 Cody N2 Diana CO Kevin BF Wai BH Mike S NH Ly HF Jake SH Melissa F2Molecular Orbital Diagram for Carbon Dimer (C2).Fill from the bottom up, with 8 electrons total.Bonding Order is 2, and it is Diamagnetic.sigma2s(2),sigma2s*...View C2 - Molecular Orbital Theory.pdf from CHM 361 at MARA University of Technology. CHAPTER 2 MOLECULAR ORBITAL THEORY FARIESHA FARHA RAMLI CONTENT • Types of Molecular Orbitals • HomonuclearMolecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of
Re: MO Diagram for C2+ and C2-
Postby saialam 1A » Sat Dec 13, 2014 5:18 pm
The resolution is C2- as a result of bond ordersWhen we draw the C2 MO, we've got the whole lot up till the PiPy Orbitlal filled, and the next orbital tht would be stuffed would be the sigma2Pz orbital. As for bond orders it is 1/2*[(#e- in bonding orbitals)-(#e- in antibonding orbitals)]Doing this, most often just C2 is 1/2*[(8)-4]=2With C2-, we are adding an e- to the standard bonding shells so: 1/2*[(9)-4)]=2.5And C2+, we remove one from the traditional bonding shell so:1/2*[(7)-4)]=1.52.5>1.Five this is the reason the C2- is more potent.
Chemistry: Molecular orbitals 2

Qualitative molecular orbital diagram for MoC. The 10 ...

Pin on chemistry

MOT: Molecular Orbital Diagrams for Li2, Li2+, Be2, B2, C2 ...

Molecular Orbital Diagram For C2 - Hanenhuusholli
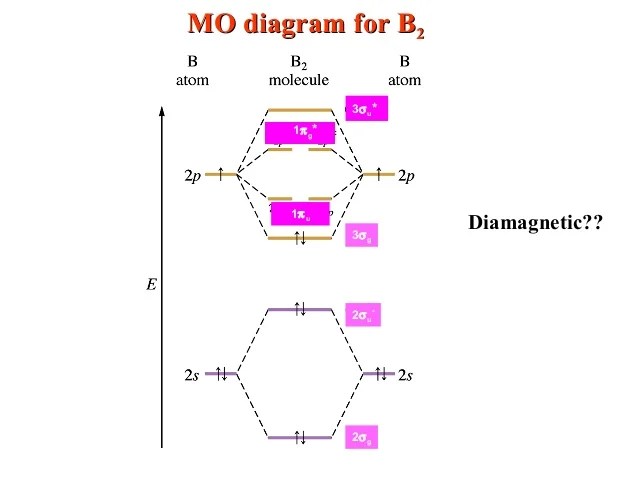
33 Molecular Orbital Diagram For C2 - Wire Diagram Source ...
C2 Molecular Orbital Diagram - Derslatnaback

Diatomic Species | MO theory | Chemogenesis

Molecular Orbital Diagram For C2 - Hanenhuusholli

32 C2+ Molecular Orbital Diagram - Wiring Diagram Database

Wiring Diagram: 13 Molecular Orbital Diagram For C2
32 C2+ Molecular Orbital Diagram - Wiring Diagram Database

C2 Molecular Orbital Diagram - Free Wiring Diagram

34 Molecular Orbital Diagram For C2 - Wiring Diagram List

C2 Molecular Orbital Diagram - General Wiring Diagram
Wiring Diagram: 13 Molecular Orbital Diagram For C2

Between N2 and N2+, which has less bond energy and why ...
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond ...

Solved: A) B) Use The Molecular Orbital Energy Diagram Bel ...

0 comments:
Post a Comment